The Nuclear Physics Group (GFN) of UCM has been working on the field of Positron Emission Tomography (PET) for more than 10 years. Our initial goal was to develop a software for image reconstruction based on a realistic physics model of the processes involved in the emission and detection of the radiation in a PET acquisition, which should be able to obtain quantitative high-quality images in a reasonable amount of time.
As physicts, when we started working on medical imaging, we were surprised by the fact that most of the images obtained from clinical and preclinical PET, CT and MRI scanners were created (reconstructed) using oversimplified, and often inaccurate methods and models. Due to the critical importance of these images for many human lives, and the high cost of these scan acquisitions, there should be no justification for not getting the best possible images from the already recorded data. If a radiologist cannot detect a tumor in a patient just because an imprecise reconstruction process made the images to be a little bit too blurry, or too noisy, or without enough contrast, it is totally unacceptable.
At GFN-UCM we developed in 2004 the PET image reconstruction software FIRST (LINK), licensed to the Spanish company Suinsa for their preclinical PET eXplore Vista scanner, and then distributed worldwide for several years by General Electric. FIRST combines a realistic model of the physics of the processes involved in PET, together with parallel computing and data compression methods. I am proud that it is still being used in many prestigious research centers in the world such as Massachusetts General Hospital and Harvard Medical School, and Johns Hopkins School of Medicine. The software has been improved over the years, especially as I used GPUs to further reduce the reconstruction time, and now it is part of the new commercially available preclinical PET scanner SuperArgus (Sedecal) (http://www.sedecal.com)
Over these years, at GFN-UCM we have been able to acomplish our goals in PET imaging:
- Development of a realistic simulation software for PET (PeneloPET) (LINK)
- Development of a fast reconstruction software for PET able to use the realistic models from simulations and produce high-quality images (FIRST and GFIRST) (Link)
- Development of data processing tools for quantitative PET Imaging (Link)
Our group is currently pushing forward the limits of PET imaging with two innovative and breakthrough projects:
- Real-Time PET imaging (LINK)
- Multiplexed PET imaging (LINK) - mPET enabled for the first time the accurate simultaneous imaging of the biodistribution of 2 different molecules in the body with preclinical and clinical PET scanners, and opened a wide range of new applications and research studies. For instance, it could be used to detect not only the presence and location of a tumor, but also measure some its relevant characteristics. This complementary information is not currently obtained, as it would require separated expensive acquisitions and several visits to already crowded hospitals and scanners. Obtaining at least two of these parameters from the same scan session would be very beneficial for the correct diagnosis and appropriate treatment selection.
Medical practice is changing rapidly, due to all the recent technological advances, towards what is called personalized or precision medicine. Treatments will be more patient-specific, and based on all the information and images gathered from the patient. We do believe that the new capabilities of PET scanners may play a significant role in this paradigm shift, as a way to obtain a more comprehensive view of the disease.
COLABORATION WITH COMPANIES:
One of our goals at GFN-UCM is to transfer the new methods and technologies developed in our group to companies developing scanners and software products so that they could reach the ultimate users: researchers all over the world (in the case of preclinical scanners) and physicians and patients (in the case of clinical scanners).
Here is the two scanners that have software designed and developed and GFN-UCM:
- Argus PET/CT Preclinical Scanner – Sedecal (Former Suinsa)
- SuperArgus - Sedecal Preclinical S.A. –
http://www.sedecal.com/sedecal.com/en/divisiones/division_prod.php?p=61&c=5&i=2#
THESIS at GFN-UCM in PET:
Master Thesis
o Pablo Galve Lahoz - Development Of A New Generation of PET Imaging Techniques. Download
o Amaia Villa Abaunza - The path towards low close CT Scans: The case of CBCT. Download
o Alejandro López Montes - Ultra Fast PET Image Reconstruction With thenPseudoinverse. Download
PhD Thesis
o Esther Vicente – Caracterization, Improvement and Design Of Preclinical PET Scanners– 2012. PhD advisors: Joaquín L. Herraiz, Juan José Vaquero y José Manuel Udías Moinelo. Download
o Khaled Abushab - Simulation and Image Reconstruction Of Clinical TOF-PET Scanners. 2013 PhD Advisors: Joaquín L. Herraiz y José Manuel Udías Moinelo. Download
o Jacobo Cal-Gonzalez - Positron Range and Prompt Gamma Modeling In PET Imaging. 2014 (LINK). PhD Advisors: Joaquín L. Herraiz y José Manuel Udías Moinelo. Download
SELECTED PUBLICATIONS at GFN-UCM in PET:
a. S. España, J.L. Herraiz, E. Vicente, J.J. Vaquero, M. Desco, J.M. Udias. PeneloPET, a Monte Carlo PET simulation tool based on PENELOPE: Features and Validation, Physics in Medicine and Biology, 2009; 54: 1723-1742. PMID: 19242053
b. J Cal-González, J. L. Herraiz, S España, P M G Corzo, J J Vaquero, M Desco and J M Udias. Positron range estimations with PeneloPET. Physics in Medicine & Biology 2013; 58: 5127. PMID: 23835700
c. K. M. Abushab, J. L. Herraiz, E. Vicente, J. Cal-González, S. España, J. J. Vaquero, B. W. Jakoby and J. M. Udías. Evaluation of PeneloPET simulations of Biograph PET/CT scanners. IEEE Trans. Nuclear Science, 2016; 63(3): 1367 – 1374. DOI: 10.1109/TNS.2016.2527789
d. J.L. Herraiz, S. España, J.J. Vaquero, M. Desco, J.M. Udías. FIRST: Fast Iterative Reconstruction Software for PET Tomography. Physics in Medicine and Biology, 2006; 51: 4547-4565. PMID: 16953042
e. J.L. Herraiz, S. España, E. Vicente, J.J. Vaquero, M. Desco, J.M. Udías. Noise and physical limits to maximum resolution of PET images. Nuclear Instruments and Meth. in Physics A, 2007.580(2):934-937.DOI: 10.1016/j.nima.2007.06.039
f. J.L. Herraiz, S. España, R. Cabido, A. S. Montemayor, M. Desco, J. J. Vaquero, and J. M. Udias. GPU-Based Fast Iterative Reconstruction of Fully 3-D PET Sinograms. IEEE Transactions on Nuclear Science, 2011; vol. 58, no. 5: 2257-2263.DOI: 10.1109/TNS.2011.2158113
g. J.L. Herraiz, E. Herranz, J. Cal-Gonzalez, L. Cusso, J.J. Vaquero, M. Desco, J.M. Udias, (2015) Automatic Cardiac Self Gating of Small-animal PET Data. Molec. Imaging and Biology, pp. 1536-1632. PMID: 26054381
h. JL Herraiz, and A. Sitek, (2015) Sensitivity estimation in time-of-flight list-mode positron emission tomography. Medical physics 42 (11), 6690-6702. PMID: 26520759
i. E. Vicente, J. L. Herraiz, S. España, E. Herranz, M. Desco, J.J. Vaquero, J.M. Udías. (2013) Improved dead-time correction for PET scanners: application to small-animal PET. Physics in Medicine and Biology. vol. 58(7): 2059-2072. PMID: 23459028
INTERNATIONAL RESEARCH PROJECTS of GFN-UCM in PET:
- “Improved Molecular Imaging by Multi-Tracer PET” Funded by Madrid-MIT m+Vision Consortium (Link) – GFN-UCM participated in this innovative program in which fellows were selected from all over the world and received funds to design, propose and lead high-impact medical imaging projects. We participated in a project together with groups from Harvard Medical School in Boston.
- “3D Assessment of Airway Liquid Absorption and Mucociliary Transport: Key Markers of Lung Disease Pathophysiology” Funded by National Institute of Heath USA (Project: R21 EB020849-01) – PI- Jose G. Venegas (Massachusetts General Hospital). (2015-2017) – GFN-UCM participate in this 2-year R21 grant using mPET to measure the absorption rate of aerosols inside the lungs.
- “Smart and self-reporting clinical nano carriers for drug delivery” PI. Jan Grimm - Memorial Sloan Kettering Cancer Center (New York) – NIH funded project 1RO1CA215700-01 – GFN-UCM participates in this 5-year grant using mPET to measure nanoparticles labeled with multiple tracers with PET scanners.
DATA PROCESSING IN POSITRON EMISSION TOMOGRAPHY
The use of high-performance reconstruction methods and accurate system models from MC simulations are not enough to guarantee high-quality and quantitative PET images. There are many other corrections that need to be considered. At GFN we have been working extensively over the last ten years in this kind of corrections:
• filling missing data in the sinograms (LINK)
• noise filtering (LINK)
• dead-time in the detectors (LINK)
• detector sensitivity normalization (LINK)
• positron range (LINK)
Our goal was to be able to obtain these corrections just from the acquired data (i.e. without additional equipment or acquisitions), as that would make them feasible in clinical scenarios.
One good example of these data-driven corrections, is a recent work (“Automatic Cardiac Self-Gating of Small-animal PET Data”) in which we proved that it is possible to detect, measure and correct for the cardiac motion in rats and mice in a preclinical PET scan just using the PET data. It was the first time this was achieved, it was considered that it was not possible due to the high frequency rate of their heart beat (around 7 beats/s for rats, and 10 beats/s per mice) (See Figure).
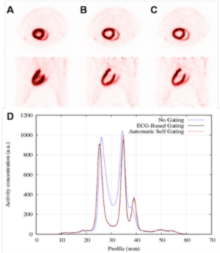 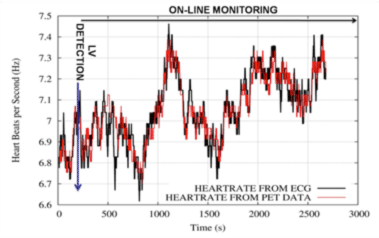
Figure (Left) (A) Transverse (above) and sagittal (below) views of a rat heart obtained with 18F-FDG PET without motion correction, (B) corrected using the information from an electrocardiogram (ECG), and (C) corrected using the proposed method. (D) Profiles along the heart in these images, are also shown. (Right) Demonstration of the hearth rate monitoring capabilities of the method proposed based only on PET, compared with the measurements obtained with the ECG.
We have also published other papers addressing different challenges for each of these corrections, obtaining a significant overall improvement of the final reconstructed images. Two students obtained their PhD in our group on these topics: PhD. Esther Vicente in 2012 -currently postdoc at Johns Hopkins University, Baltimore, MD, USA-) and (Jacobo Cal-Gonzalez in 2014 - currently postdoctoral fellow at the Center for Medical Physics and Biomedical Engineering at the Medical University of Vienna in Austria).
REFERENCIAS:
1) J.L. Herraiz, E. Herranz, J. Cal-Gonzalez, L. Cusso, J.J. Vaquero, M. Desco, J.M. Udias, (2015) Automatic Cardiac Self Gating of Small-animal PET Data. Molec. Imaging and Biology, pp. 1536-1632. PMID: 26054381
2) JL Herraiz, and A. Sitek, (2015) Sensitivity estimation in time-of-flight list-mode positron emission tomography. Medical physics 42 (11), 6690-6702. PMID: 26520759
3) E. Vicente, J. L. Herraiz, S. España, E. Herranz, M. Desco, J.J. Vaquero, J.M. Udías. (2013) Improved dead-time correction for PET scanners: application to small-animal PET. Physics in Medicine and Biology. vol. 58(7): 2059-2072. PMID: 23459028
Multiplexed Emission Tomography (mPET)
PET is an invaluable imaging technique for locating tumors in-vivo by providing images of glucose metabolism (with 18F-FDG). Additional PET images of other administered molecular radiotracers could provide important complementary information of the tumor, and a more comprehensive view of the disease. Nevertheless, PET lacks the capacity of distinguishing signals from different radiotracers, (as they all end-up emitting 511 keV gamma-rays), and therefore the use of additional tracers would require a second visit, days later, for a second scan. This option is not only expensive but also inaccurate, as significant misalignments between scans may occur.
In the last few years, we have been leading a project called multiplexed PET (or mPET), that enables simultaneous imaging of two radiotracers in a single PET scan. We have proved that the detection, recovery and use of double and triple coincidences in a PET scan (i.e. 2 or 3 gamma rays detected simultaneously) instead of only using standard double coincidences, allows the differentiation of tracers labeled with PET isotopes (such as, 82Rb, 78Br, or 124I) that emit prompt gamma rays together with the positrons, from those labeled with pure positron emitters (such as 18F). Our experiments have shown that recovered separated images from a simultaneous dual-tracer PET acquisition are comparable to those obtained in sequential single tracer scans, and we have 4 PCT patents on this technology.
We have used preclinical PET/CT scanners in Madrid and Boston to simultaneously acquire molecular information from two different radiotracers in living rodents, for oncological and neurological applications. We obtained a NIH grant with Prof. Jose Venegas at Massachusetts General Hospital to evaluate its performance in clinical scanners. mPET allowed studying the difference in the clearance of two aerosols from the lungs of mini pigs with unprecedented resolution and sensitivity. The integration of this technology in clinical PET/CT and PET/MRI systems will open new perspectives in molecular imaging and holds the potential to advance towards personalized medicine, by enabling the measurement of a range of disease aspects in a single scan.
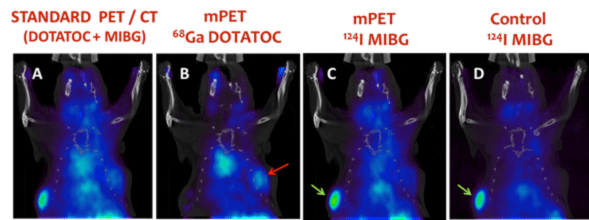
Figure 4. In vivo mPET imaging example using a 28-g mouse with 2 tumors implanted in the flanks. Each tumor express different receptors which are targeted by the specific tracers injected to the animal: 124I -MIBG (400 µCi) + 68Ga-DOTATOC (300 µCi). (A) Image obtained with a standard PET acquisition (30’), showing the sum of the distribution of both tracers (B, C) Separated images of each radiotracer obtained with mPET from the same data. The image obtained for 68Ga –DOTATOC shows uptake in the right tumor (red arrow) and no uptake in the left tumor (green arrows) while the 124I –MIBG shows the opposite behavior. (D) Control single-tracer 124I-MIBG image acquired 2 hours before the images shown in A-C. It is comparable to the image shown in C.
REFERENCES:
1) J.L. Herraiz, S. C. Moore, M. Cervo, S. España, J. Ruiz-Cabello, E. Lage. “Multiplexed PET in Clinical Scanners Based on Triple Coincidences”, IEEE Nuclear Science Symposium & Medical Imaging Conf., Seattle, USA, 2014, #M06-7.
2) E. Lage, V. Parot, S. C. Moore, A. Sitek, J.M. Udias, S.R. Dave, M-A Park, J.J. Vaquero, J.L. Herraiz
(2015) Recovery and Normalization of triple coincidences in PET. Medical Physics. 42(3), 1398-1410. PMID: 25735294
3) J. Cal-Gonzalez, E. Lage, E. Herranz, E. Vicente, J.M. Udias, S. C. Moore, M-A Park, S.R. Dave, V. Parot, J.L Herraiz (2015) Simulation of triple coincidences in PET. Physics in Medicine and Biology, 60, 117-136. PMID: 25479147
|


